Pedro F. Gouveia, Joana Costa, Pedro Morgado, Ronald Kates, David Pinto, Carlos Mavioso, João Anacleto, Marta Martinho, Daniel Simões Lopes, Arlindo R. Ferreira, Vasileios Vavourakis, Myrianthi Hadjicharalambous, Marco A. Silva, Nickolas Papanikolaou, Celeste Alves, Fátima Cardoso, Maria João Cardoso.
KEYWORDS
Breast cancer. Breast conservative surgery . 3D breast model . Augmented reality
ABSTRACT
Introduction: Innovations in 3D spatial technology and augmented reality imaging driven by digital hightech industrial science have accelerated experimental advances in breast cancer imaging and the development of medical procedures aimed to reduce invasiveness. Presentation of case: A 57-year-old post-menopausal woman presented with screen-detected left-sided breast cancer. After undergoing all staging and pre-operative studies the patient was proposed for conservative breast surgery with tumor localization. During surgery, an experimental digital and non-invasive intra-operative localization method with augmented reality was compared with the standard pre-operative localization with carbon tattooing (institutional protocol). The breast surgeon wearing an augmented reality headset (Hololens) was able to visualize the tumor location projection inside the patient’s left breast in the usual supine position. Discussion: This work describes, to our knowledge, the first experimental test with a digital non-invasive method for intra-operative breast cancer localization using augmented reality to guide breast conservative surgery. In this case, a successful overlap of the previous standard pre-operative marks with carbon tattooing and tumor visualization inside the patient’s breast with augmented reality was obtained. Conclusion: Breast cancer conservative guided surgery with augmented reality can pave the way for a digital non-invasive method for intra-operative tumor localization.
1. INTRODUCTION
Breast conservative surgery (BCS) combined with radiotherapy has become the treatment of choice for the majority of women presenting with early breast cancer [1]. The aim of BCS is to achieve optimal long-term local control, with free margins after tumor excision and a good cosmetic outcome. Due to widespread screening an important percentage of early breast cancers are nonpalpable. As a consequence, tumor localization is needed to assist the surgeon during surgery. Current modalities for pre-operative localization of breast cancer lesions are all invasive procedures requiring imaging guidance: wire-guided, carbon tattooing, or more recently, radioactive seed localization, radio-occult lesion localization and magnetic seeds [2].
Innovations in 3D spatial technology and augmented reality (AR) driven by digital high-tech industrial science have accelerated exploratory research in breast cancer imaging. Although extensive literature exists on 3D finite elements simulation and breast tissue modeling, few papers have addressed breast magnetic resonance imaging (MRI) to 3D scan fusion to simulate a real breast [3–8]. Recently, a patient-specific 3D digital breast model integrating the breast torso and tumor location was created and validated with a MRI to 3D surface scan fusion algorithm [9]. The spatial computing applied to a breast cancer patient, merging digital and physical anatomic structures of the breast with tumor included in a digital 3D breast model, can be visualized through AR in the operating theater.
The objective of this work is to report a clinical case of a breast cancer conservative guided surgery with AR.
2. PRESENTATION OF CASE
2.1. Case History
A 57-year-old post-menopausal woman presented with a screen-detected left sided breast cancer. Inferior periareolar microcalcifications were present with an estimated mammography extension of 17 mm associated to a 15 mm dystrophic area on ultrasound (US). A 29 mm breast MRI nodular lesion was observed. A guided vacuum-assisted biopsy with clip placementwas performed showing an invasive breast carcinoma of non-special type (NST), grade 2, ER and PR of 100%, HER2 negative with a Ki67 of 20% (1 fragment in 10), associated with intermediate grade ductal carcinoma in situ (DCIS). Bilateral physical examinationwas negative for palpable lesions and axillary lymph nodes.
2.2. Experimental protocol description to produce a patient-specific 3D digital breast model (phantom model)
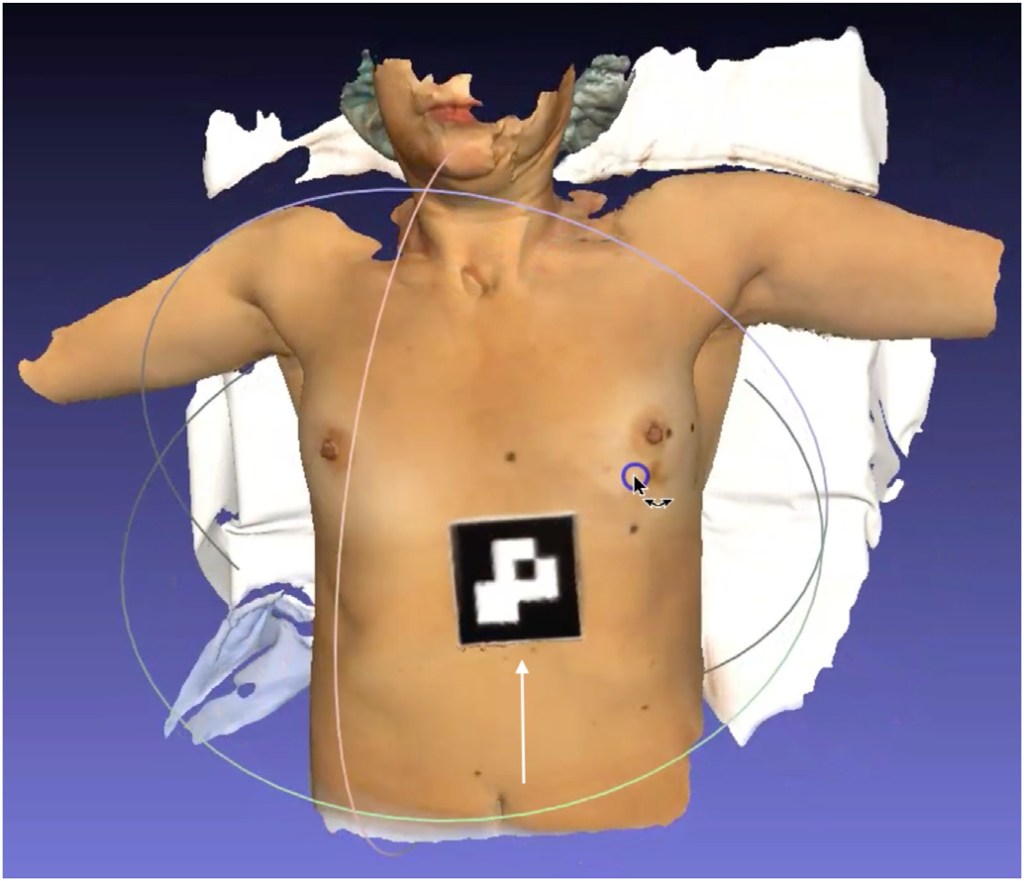
Three breast surface markers (BSM) were applied on the patient’s breasts (infra-mammary sulcus) along with a 10 cm fiducial marker below the xiphoid process (Fig. 1). These reference points were annotated with a black permanent marker at the patient’s skin. A 3D surface scan of the patient was performed in the supine position with arms at 90 degrees (Fig. 1), capturing the size and shape of both breasts and torso using a Go!Scan 3D handheld by CreaformTM. After 3D surface scan, fish-oil capsules were fixed on the three BSM marks for MRI acquisition. A contrast-enhanced MRI was performed with the patient in prone position and markers in place. The institutional breast MRI acquisition protocol was modified to include a final 5-min Dixon sequence with the phased array torso MRI coil and the patient in the supine position with arms along the body (Fig. 2).
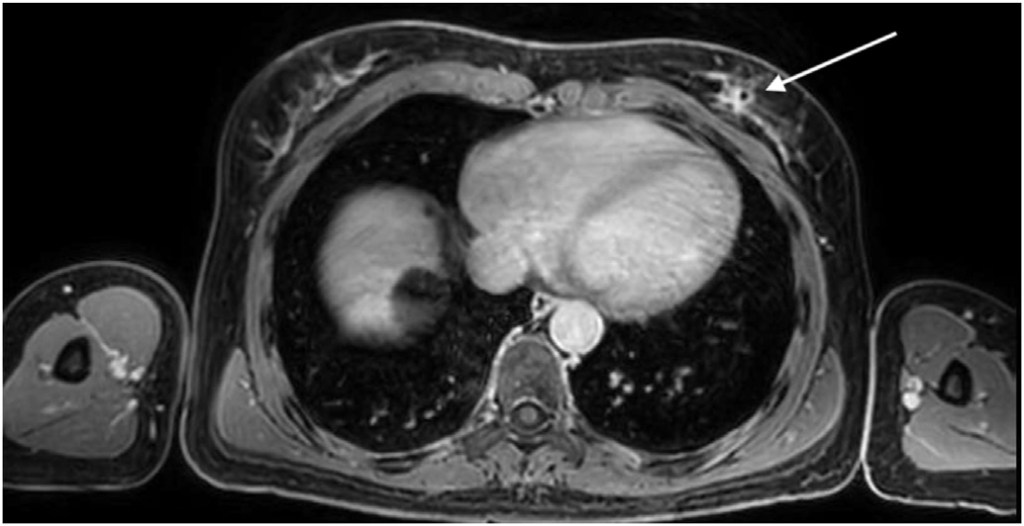
Annotation, segmentation and volume computation of the MRI tissue portions (with Dixon sequence in the supine position) were performed and validated by two radiologists using Horos R software v2.4.0 (breast contour, breast tissue including malignant tumor and latissimus dorsi muscle anterior border). Breast surface markers were manually annotated on the 3D surface scan (black permanent marker) and MRI data (fish-oil capsules).
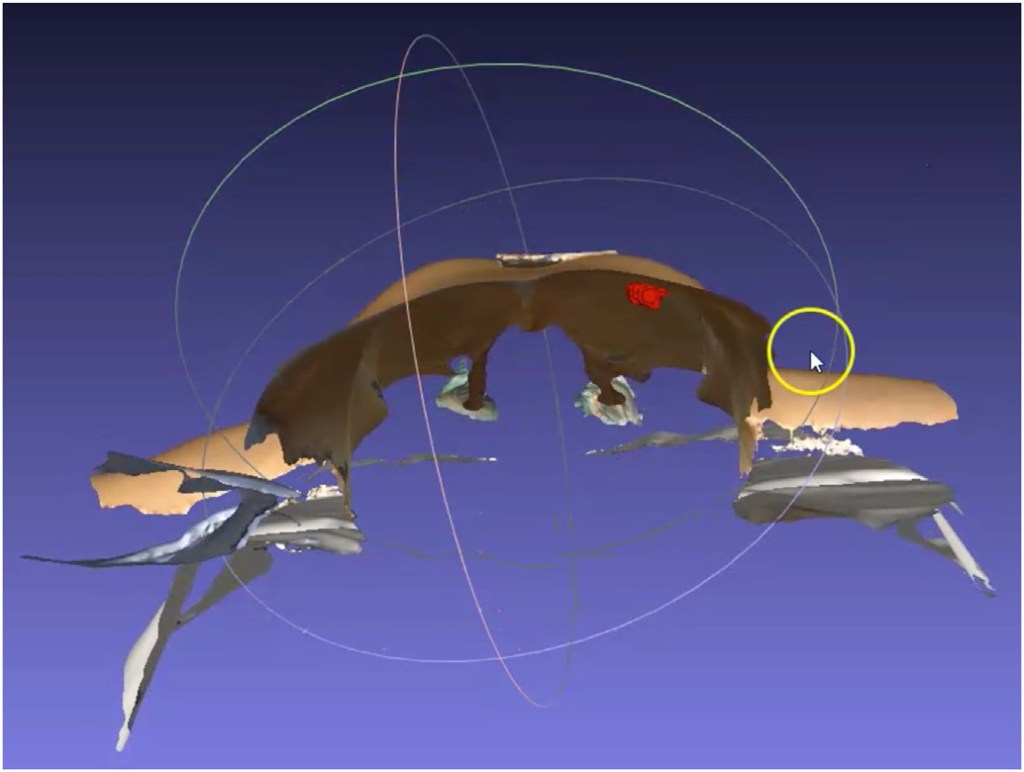
Breast MRI/3D scan fusion was accomplished with simple overlap between both modalities, including tumor, using BSM to perform spatial alignment between both modalities (Fig. 3). A patient-specific 3D digital breast model was processed and converted to a point cloud breast 3D model, referred hereafter as phantom model (Fig. 5), ready to be uploaded to an AR headset: Hololens 1.


2.3. Surgery with AR headset
Standard pre-operative localization with carbon tattooing under ultrasound guidance was performed targeting the nodular lesion and the clip (microcalcifications). Preoperative markings were drawn on the patient skin in the standing position. During surgery, an experimental digital and non-invasive intra-operative localization method with AR was compared with the standard preoperative localization with carbon tattooing. The breast surgeon wearing an AR headset (Fig. 4), synchronized the patient-specific 3D digital breast model (viewed through the Hololens) with the real patient lying down in the operating theatre bed, projecting the tumor location inside the left breast (Fig. 5).
A breast conservative surgery with sentinel lymph node biopsy through an inferior peri-areolar incision was performed, followed by defect repair with local glandular flaps.
Pathology: 17 mm tumor was removed consisting of intermediate grade DCIS with free margins and one negative sentinel lymph node. No invasive cancer was found. Final TNM staging was pTis pN0(sn)cM0.
3. DISCUSSION
Surgeons need to be able to correlate 2D radiological images with the real location of the tumor within the patients breast for surgical planning, both pre and per-operatively. Despite all preoperative and intra-operative localization techniques, accuracy is still not perfect, and the process requires additional exams and invasive procedures in the pre-operative setting or expertise in US techniques in case of an intra-operative setting.
The diagnostic breast cancer workflow includes different imaging modalities acquired in different positions and with different breast compressions and consequent deformations. As breast cancers are diagnosed more frequently at an early stage and most of them are non-palpable, localization techniques are frequently needed: wire-guided, carbon tattooing, radioactive seed localization, radio-occult lesion localization and magnetic seeds are considered as standard. However, among these techniques, none has proven superiority in terms of reducing positive margins [2]. Worldwide, re-excision rates following breast cancer conservative surgery exhibit wide variability. Re-operation rates range from 0% to 70% (by individual surgeons) in the United States [10], from 17% to 56% in Canada [11], and from 12 to 30% [12] in England.
Several strategies have been proposed to improve breast cancer surgical outcomes. Preoperative contrast-enhanced breast MRI has emerged as a solution, but neither the COMICE [13] nor the MONET [14] trial found an associated benefit. Additional studies are needed to further exploit additional MRI information and techniques to be applied in the surgical theater [15]. Intraoperative US (as an additional tool to standard localization techniques) has been used successfully to some extent to reduce positive margins, therefore reducing re-operation rates [16,17]. However, the use of intraoperative US requires a level of expertise beyond the standard for breast surgeons and could also be time-consuming within the surgical theater. These considerations strongly suggest the need for development of a novel methodology to enhance the surgeon’s visualization for tumor localization and consequently to improve surgical clinical outcomes.
This work describes a successful experimental test with a digital non-invasive method for intra-operative breast cancer localization. A patient-specific 3D digital breast model was processed and viewed through AR to guide a breast cancer conservative surgery. In this case an overlap of the previous standard pre-operative marks with carbon tattooing and tumor visualization inside the patient’s breast with AR was obtained (Fig. 5).
Perkins et al. [18] proposed a similar approach, but with a different methodology for surgical planning and not suitable for live surgery: they projected a breast MRI 3D hologram registered with the patient in the supine position and applied it with six fiducial markers. Our methodology incorporates a breast MRI to 3D surface scan fusion in order to produce a complete patient-specific 3D digital breast model (phantom model) with only one fiducial marker and three infra-mammary breast surface markers, used during both imaging modalities registration to optimize image synchronization. The diagnostic breast MRI in prone position is performed with a final 5-min Dixon image sequence in the supine position. The institutional protocol needed to be adapted with patient repositioning from prone to supine during the MRI registration. Breast MRI to 3D surface scan fusion algorithms with pose transformation simulation from prone to supine [9] can potentially leverage this technology without the need to change diagnostic institutional breast MRI registration protocols. A two steps prospective clinical trial (pre-clinical plus clinical steps) was designed and will start recruiting during 2021 to address this limitation.
To improve AR user experience applied for breast cancer conservative surgery, 3D space camera anchors installed in the roof of the operating theatre could be used to perform real-time 3D mapping of the patient in the operating theater, replacing handheld 3D surface scan devices.
3.1. Conclusion
Breast cancer conservative guided surgery with an AR headset, using 3D digital breast models, could pave the way for a digital, non-invasive method for intra-operative tumor localization. The proposed solution has the potential to improve the surgeon’s visualization of the tumor while improving patient’s quality of life (no pain, no anxiety, no bleeding).
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Fatima Cardoso reports personal financial interest in form of consultancy role for: Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Medscape, Merck-Sharp, Merus BV, Mylan, Mundipharma, Novartis, Pfizer, Pierre-Fabre, prIME Oncology, Roche, Samsung Bioepis, Sanofi, Seagen, Teva.
DECLARATION OF COMPETING INTEREST
No conflict of interest and financial relationships relevant to the content of this article have been disclosed by any of the other authors.
REFERENCES
[1] van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143e50.
[2] Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol 2013;2013: 793819.
[3] Lee A, Schnabel J, Rajagopal V, Nielsen P, Nash M. Breast image registration by combining finite elements and free-form Deformations 2010.
[4] Reece GP, Merchant F, Andon J, Khatam H, Ravi-Chandar K, Weston J, et al. 3D surface imaging of the human female torso in upright to supine positions. Med Eng Phys 2015;37:375e83.
[5] Bjoern E, Vasileios V, John HH, Sven K, Cristian L, Thomas B, et al. Surface driven biomechanical breast image registration. ProcSPIE 2016.
[6] Vavourakis V, Eiben B, Hipwell JH, Williams NR, Keshtgar M, Hawkes DJ. Multiscale mechano-biological finite element modelling of oncoplastic breast surgery-numerical study towards surgical planning and cosmetic outcome prediction. PloS One 2016;11:e0159766.
[7] Salmon R, Nguyen T, Moore L, Bass B, Garbey M. Multimodal imaging of the breast to retrieve the reference state in the absence of gravity using finite element Modeling 2017.
[8] Duraes M, Crochet P, Pages E, Grauby E, Lasch L, Rebel L, et al. Surgery of nonpalpable breast cancer: first step to a virtual per-operative localization? First step to virtual breast cancer localization. Breast J 2019;25:874e9.
[9] Bessa S, Gouveia PF, Carvalho PH, Rodrigues C, Silva NL, Cardoso F, et al. 3D digital breast cancer models with multimodal fusion algorithms. Breast 2020;49:281e90.
[10] McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, et al. Variability in reexcision following breast conservation surgery. J Am Med Assoc 2012;307:467e75.
[11] Porter G, Wagar B, Bryant H, Hewitt M, Wai E, Dabbs K, et al. Rates of breast cancer surgery in Canada from 2007/08 to 2009/10: retrospective cohort study. CMAJ Open 2014;2:E102e8.
[12] Jeevan R, Cromwell DA, Trivella M, Lawrence G, Kearins O, Pereira J, et al. Reoperation rates after breast conserving surgery for breast cancer among women in England: retrospective study of hospital episode statistics. BMJ 2012;345:e4505.
[13] Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010;375:563e71.
[14] Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET – randomised controlled trial. Eur J Canc 2011;47:879e86.
[15] Sardanelli F, Trimboli RM, Houssami N, Gilbert FJ, Helbich TH, Alvarez Benito M, et al. Solving the preoperative breast MRI conundrum: design and protocol of the MIPA study. Eur Radiol 2020.
[16] Krekel NM, Haloua MH, Lopes Cardozo AM, de Wit RH, Bosch AM, de Widt- Levert LM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol 2013;14:48e54.
[17] Spick C, Baltzer PA. Diagnostic utility of second-look US for breast lesions identified at MR imaging: systematic review and meta-analysis. Radiology 2014;273:401e9.
[18] Perkins S, Lin M, Srinivasan S, Wheeler A, Hargreaves B, Daniel B. A mixedreality system for breast surgical Planning2017.
