E.-A. Bonci, J. Correia Anacleto, M.-J. Cardoso
KEYWORDS
Breast cancer . Breast conserving therapy . Oncoplastic surgery . Reconstructive breast surgery de-escalation therapy .
ABSTRACT
Simple breast conservation surgery (sBCS) has technically advanced onto oncoplastic breast procedures (OBP) to avoid mastectomy and improve breast cancer patients’ psychosocial well-being and cosmetic outcome. Although OBP are time-consuming and expensive, we are witnessing an increase in their use, even for cases that could be managed with sBCS. The choice between keeping it simple or opting for more complex oncoplastic procedures is difficult. This review proposes a pragmatic approach in assisting this decision.
Medical literature suggests that OBP and sBCS might be similar regarding local recurrence and overall survival, and patients seem to have higher satisfaction levels with the aesthetic outcome of OBP when compared to sBCS. However, the lack of comprehensive high-quality research assessing their safety, efficacy, and patient-reported outcomes hinders these supposed conclusions. Postoperative complications after OBP may delay the initiation of adjuvant RT. In addition, precise displacement of the breast volume is not effectively recorded despite surgical clips placement, making accurate dose delivery tricky for radiation oncologists, and WBRT preferable to APBI in complex OBP cases.
With a critical eye on financial toxicity, patient satisfaction, and oncological outcomes, OBP must be carefully integrated into clinical practice. The thoughtful provision of informed consent is essential for decision-making between sBCS and OBP. As we look into the future, machine learning and artificial intelligence can potentially help patients and doctors avoid postoperative regrets by setting realistic aesthetic expectations.
Abbreviations
AI – artificial intelligence
APBI – accelerated partial breast irradiation
BCS – breast conservation surgery
sBCS – simple breast conservation surgery
BIS – body image scale
BRESO – European Breast Surgical Oncology Certification
CI – confidence interval
DFS – disease-free survival
LR – local recurrence
OBP – oncoplastic breast procedures
OR – odds ratio
OS – overall survival
QoL – quality of life
RR – risk ratio
WBRT – whole breast radiation therapy
1. INTRODUCTION
Breast cancer incidence varies by region, but the disease remains a global health issue, particularly among industrialised countries. In 2020, an estimated 2.26 million new cases and 685,000 deaths were attributed to breast cancer, making it the most prevalent form of female malignancy worldwide [1]. Mortality rates have significantly declined over the past two decades due to improved screening practices and increased access to different treatment options [2]. According to recent studies in early breast cancer [3,4], when treatment modalities like surgery, radiotherapy, and systemic therapy are combined, an overall survival (OS) of over 90% and a locoregional control rate of over 95% can be attained at five years. Consequently, the affected population includes a significant number of long-term survivors that tend to have high expectations about positive aesthetic and psychological outcomes.
The surgical management of patients has gradually changed from radical mastectomy to breast conservation surgery (BCS), followed by adjuvant radiotherapy to treat cancer while preserving the breast [5]. OS rates following the breast-conserving approach have been documented to be comparable or even better to those following mastectomy [6–13]; thus, BCS has primarily taken the place of total mastectomy in recent years. On one hand, patients with BCS have demonstrated better cosmetic outcomes and quality of life (QoL) than those with mastectomy [14–19]; on the other, it has been shown that poor cosmesis and deformity after BCS have a negative impact on QoL, as well as psychosocial and sexual function [20–26]. Skin or nipple-areola complex retraction, delayed radiotherapy side effects, and breast asymmetry are the most common residual deformities reported in up to one-third of patients with BCS [27–29].
BCS has technically advanced to what are known as oncoplastic breast procedures (OBP) to avoid the above mentioned pitfalls, improving psychosocial well-being of patients and cosmetic outcome while upholding the oncologic principle of complete tumour excision. The modern definition of oncoplastic surgery is diversified and varies from “A form of breast conservation surgery that includes oncologic resection with a partial mastectomy, ipsilateral reconstruction using volume displacement or volume replacement techniques with possible contralateral symmetry surgery when appropriate” [30] to a more broad definition including also mastectomy, reconstruction and additional symmetrisation procedures either immediately after the procedure or at a later date [31]. Nonetheless, we shall limit our review to OBP in BCS and abstain from thoroughly examining the complex topic of mastectomy with various forms of breast reconstruction. Oncoplastic surgery for BCS can be categorised into levels I and II. When less than 20% of the total breast volume is expected to be removed, breast deformities are prevented with level I techniques (simple local reshaping – simple breast conservation surgery [sBCS]), while level II techniques (reshaping the breast parenchyma and reducing the skin envelope – OBP) are used in cases where resection of more than 20% of the breast volume is anticipated [32].
Over the past three decades, OBP gained wider acceptance worldwide, being routinely offered in a growing number of breast centres. The oncoplastic approach had the largest relative growth, nearly quadrupling from 2007 to 2014 in a review of 10,607 breast cancer surgeries [33]. Although oncoplastic surgery can be a viable option for many patients with breast cancer, careful selection is essential when evaluating potential candidates. Patients with a high tumour-to-breast ratio who would suffer significant deformities following sBCS are the main candidates for OBP. Numerous studies suggest that using OBP may have several advantages [25,34–41].
• tumour excision with safe margins and reduced need for re-excision;
• good to excellent cosmetic results, even in patients with locally advanced breast cancer;
• avoids total mastectomy and breast reconstruction, resulting in lower complication and morbidity rates.
Compared with sBCS, surgical techniques used in OBP entail wider incisions, additional tissue mobilisation, and possible flap reconstruction [42,43]; involved procedures are more complex, time-consuming and expensive. While complex reconstructions and therapeutic mammoplasties may deliver improved cosmetic results, their higher cost and chance of complications have generated concern over the toxic effects that OBP might have [44,45]. Consequently, oncoplastic surgery has been the subject of numerous studies, the majority being retrospective [23,46–48]. Although some have shown encouraging results, several authors referring to OBP as a new standard of care, these techniques were not validated using randomised studies and present issues concerning safety, local and systemic control, delayed start of adjuvant treatments due to increased rate of complications, and cost-effectiveness. Moreover, standardised and robust data on aesthetic outcomes and QoL comparing OBP versus sBCS are lacking [49,50], even though oncological safety of oncoplastic techniques has been documented using large retrospective data collections [36,43,51,52].
OBP rates have dramatically increased despite ample breast-screening programmes and optimised neoadjuvant strategies; up to 50% of women are offered this approach even when their cancer can be managed with sBCS [33,53–55]. A French nationwide survey reported that only 10–15% of BCS cases required level II OBP [56]. This poses the question: why are advanced breast oncoplastic techniques increasingly used when sBCS can also result in an excellent aesthetic outcome? When de-escalating from mastectomy to BCS, are we escalating from sBCS to OBP?
With the increasing demand for less invasive breast cancer treatments, it is essential to examine the current literature on the oncological and aesthetic outcomes, safety, and costs of OBP, mainly when compared to sBCS, and to propose a pragmatic approach to help deciding between them.
2. WHO REALLY NEEDS OBP INSTEAD OF sBCS?
2.1. The patient’s perspective
As cancer patients stand in front of their surgeon, they may feel the weight of their diagnosis and often accept the clinician’s proposal without hesitation due to strong faith in the doctor’s expertise and judgement.
The choice between sBCS or more complex OBP can be straightforward when the patient, for example, has large and ptotic breasts and has been considering breast reduction for a long time. However, the choice might be more difficult if breast reduction is offered to a patient who has never considered this and needs to decide between OBP and sBCS, with less operative time, faster recovery, and lower cost.
Patients often encounter difficulty in navigating the complexities of a surgical decision, prompting the surrender of choice to their surgeon. Unfortunately, this can lead to discontent with the surgical outcome due to mismatched expectations between them and the surgeon.
2.2. The surgeon’s perspective
A surgeon’s decision between sBCS or OBP for a patient candidate for breast conservation is influenced by many factors, including locoregional staging, location, shape and tumour-to-breast ratio, size of the breasts, as well as biological age, comorbidities, along with social and economic conditions.
The variability that leads to this decision is immense and includes the surgeon’s skills and economic issues in addition to tumour and patient factors. Usually, the shared decision-making process is unbalanced with what the surgeon thinks is best for the patient, especially if the patient is satisfied with whatever has been proposed.
The traditional acceptance of a physician’s treatment plan, “the doctor knows best”, is being challenged as individuals now have more access to information than ever before. In some cases, this can help provide an extra layer of insight and lead to better decisions; however, it could also be problematic if the surgeon is not well-versed in oncoplastic surgery or needs proper qualifications.
Involving patients in the decision-making process increases the likelihood of mutually agreed-upon expectations and satisfaction with the outcome. Patients should be sufficiently knowledgeable about their treatment plans to make educated decisions that are likely to result in minimised regret.
3. WHICH FACTORS CAN INFLUENCE THE CHOICE BETWEEN OBP AND sBCS?
3.1. Surgeon’s training/expertise
OBP should incorporate preoperative assessment of patient’s anatomy and expectations as well as an evaluation of the amount of tissue that needs to be removed and how it will be dealt with, creating a surgical plan tailored to remove the tumour while maintaining or even improving the appearance [57]. There is a learning curve to this approach before becoming fully competent in performing OBP with satisfactory results. Klimberg [58] suggests that for those just getting started in oncoplastic surgery, the best option would be to begin with level I procedures, simple enough for any surgeon. More complex level II techniques can be introduced as skill develops and should require specialised training [59].
Currently, no country offers training in breast surgery as a stand-alone discipline. Instead, medical professionals must obtain certification through General Surgery, Gynaecologic, or Plastic Surgery residencies over 4–6 years. These training programmes include an often brief segment of specialised knowledge on breast diseases – sometimes lasting only months and occurring out of formal multidisciplinary teams [60]. However, in the past two decades, fellowships dedicated to Breast Surgical Oncology have widely spread worldwide. These programmes provide future surgeons with a comprehensive understanding of related medical fields, such as medical oncology, pathology, radiology, radiation oncology, rehabilitation, and survivorship care. These are all essential elements in providing high-quality cancer treatment [61]. The United States of America, the United Kingdom, Australia, and New Zealand have been at the forefront of training highly-skilled specialists through 1 or 2-year fellowships. Still, these opportunities remain scarce within European countries, translating into an increase in generalist treatment for women with breast cancer [60]. The European Breast Surgical Oncology Certification (BRESO), an initiative founded in 2019, is striving to improve breast surgery education, mainly focusing on honing the skills of oncoplastic techniques. A reliable and uniform standard across European countries would ensure that all healthcare professionals could provide their patients with adequate quality care [62,63].
Currently, OBP have yet to be firmly established in therapeutic protocols globally; the lack of implementation can largely be attributed to a dearth of standardised training programmes, inadequate access or challenging collaborations with plastic surgery teams, insufficient assistance from adjunct disciplines (medical and radiation oncology), increased operating time, absence of appropriate reimbursement and inadequate scientific evidence [14,25,26,58,64,65].
3.2. The complexity of surgery
According to a study published by Adamson et al. [66], patients undergoing level II OBP should be aware of potential postoperative complications, which can occur at a rate of approximately 25%. The most common postoperative issues are wound healing, infection, fat necrosis, and hematoma or seroma formation (see Table 1). Furthermore, multivariate regression analysis revealed that those with a higher body mass index or diabetes might have an increased risk of postoperative complications requiring further operative treatment.
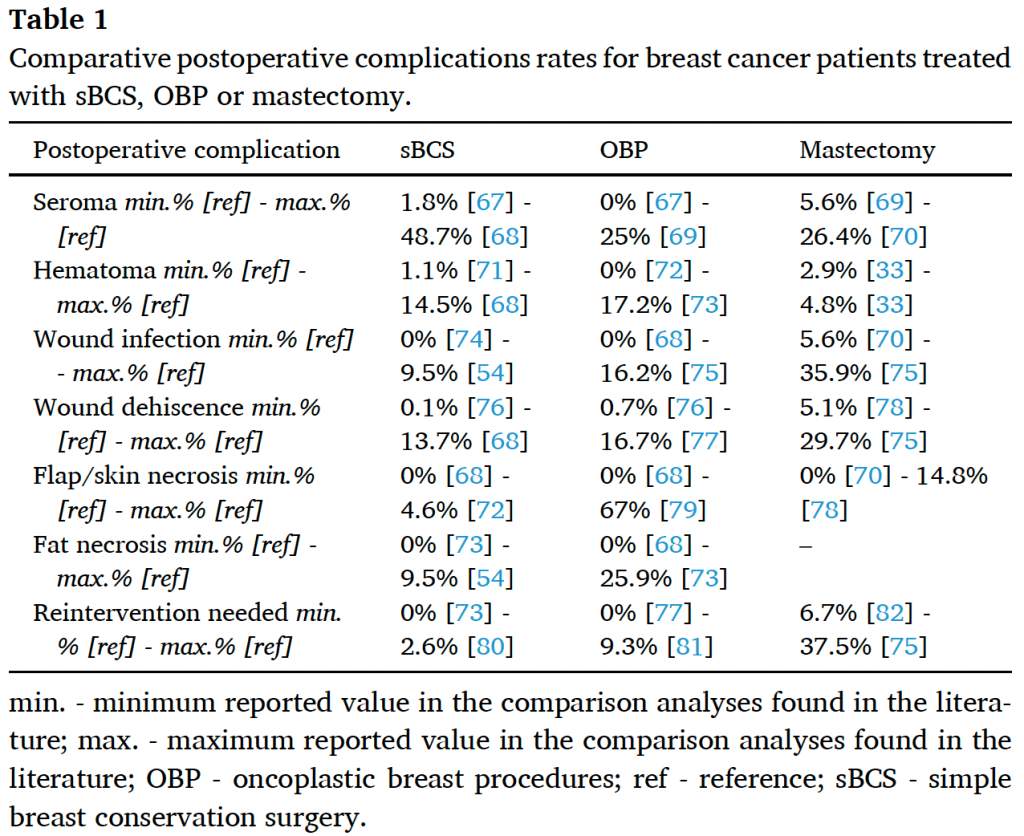
One of the largest meta-analyses [83] confirmed these results, showing that OBP, compared to sBCS, can lead to an increased number of women with at least one postoperative complication (risk ratio [RR] 1.19, 95% confidence interval [CI] 1.10 to 1.27; 20 studies, 118,005 participants). Despite very low certainty of evidence, this might be clinically plausible due to the more extensive resections performed and the novelty of the technique compared to sBCS. On other hand, compared to mastectomy with or without reconstruction, the same meta-analysis concluded that OBP might decrease postoperative complication rates (RR 0.49, 95% CI 0.45 to 0.54; 5 studies, 4973 participants; very low-certainty evidence). While bias and inconsistency challenge such conclusions, further high-quality research is needed to obtain more reliable data.
3.2.1. Delays in adjuvant treatment
A recent meta-analysis suggested that delays between surgery and radiation is associated with higher local relapse rate [84]. Published studies show that the number of days before the start of adjuvant therapy seem to increase when using OBP compared to sBCS [73,83,85]. The latest Cochrane analysis [83] showed that using OBP instead of sBCS increased the number of days deemed to start adjuvant therapy, but only for radiotherapy, from 7 to 12 days. It is therefore crucial to consider that delays could impact patient care and oncologic outcomes.
3.2.2. Re-excisions and conversion to mastectomy
OBP have been associated with a reduced risk of re-excision and fewer positive surgical margins than sBCS [14]. Nonetheless, OBP can often complicate subsequent re-excisions, potentially requiring mastectomy to ensure complete tumour removal.
In a study of 277 level II OBP performed at The Paris Breast Centre between 2004 and 2013 [86], positive surgical margins were found in 33 cases (11.9%), and mastectomy necessary in 2/3 of these. A meta-analysis of approximately 8500 patients, published by Losken et al. [25], analysing sBCS and OBP, revealed that positive tumour margins were significantly lower after an oncoplastic approach (21% vs 12%, P < 0.0001), with a decreased need for re-excision (14.6% vs 4%, P < 0.0001). However, subsequent re-excisions in the OBP group led to a higher mastectomy rate (3.79% vs 6.5%, P < 0.0001). A Cochrane meta-analysis [83] confirmed that, compared to sBCS, OBP might lead to reduced re-excision rates due to positive surgical margins (RR 0.76, 95% CI 0.69 to 0.85; 38 studies, 13,341 participants; very low-certainty evidence). On other hand, a Danish population-based national study of 18, 188 patients [87], in a multivariable analysis, showed a decreased risk of undergoing re-excision for positive surgical margins after OBP (OR 0.80, 95% CI 0.72 to 0.88), but also a lower likelihood of conversion to mastectomy than those who underwent sBCS (OR 0.69, 95% CI 0.58 to 0.84). According to the authors, several factors may influence the results of the previous published meta-analyses, such as a lack of accounting for confounders or exclusion of patients with carcinoma in situ.
Factors associated with increased likelihood of re-excision following OBP include increased body mass index, microcalcifications, tumour multifocality, extensive ductal carcinoma in situ, and invasive lobular disease [86–89]. The complexity of re-excision, decreased locating accuracy concerning the initial tumour bed, is likely why mastectomies are more commonly used in cases involving surgical margins after an oncoplastic approach. Additionally, large excisions can reduce breast size to the extent that further excisions may result in poor cosmesis [86].
3.2.3. Oncologic outcomes
All studies published until August 2020 that evaluated 178,813 women were analysed in a comprehensive Cochrane review [83]. Comparing OBP to sBCS, there may be little to no differences in local recurrence (LR)-free survival, LR rate, disease-free survival (DFS) and OS based on evidence with a very low level of certainty. As most studies did not account for confounding clinicopathological factors, the evidence could be considered speculative.
3.3. Radiotherapy
3.3.1. Postoperative radiation challenges
Studies have shown that breast cancer recurrence is most likely to occur near the original tumour site, indicating a propensity for residual malignant cells near the primary lesion [7,90–93]. To maximise local control in BCS, adjuvant radiotherapy must target the tumoural bed area [4]. However, scientific evidence about the effectiveness of adjuvant irradiation in controlling recurrence after OBP is limited [85].
A study of 965 patients with breast cancer, published by Borm et al. [85], showed that immediate oncoplastic surgery and adjuvant radiotherapy yielded similar local control rates as sBCS. Furthermore, the most recent Cochrane review [83], conducted with 78 non-randomised cohort studies, including 178,813 women, concluded that there was little to no difference between LR, DFS, or OS post-treatment between sBCS and OBP. It might seem that tissue reshaping during oncoplastic surgery does not meaningfully affect adjuvant radiation therapy outcomes. Yet, due to low certainty evidence, these are only preliminary conclusions.
3.3.2. Accelerated partial breast irradiation (APBI) vs whole breast radiation therapy (WBRT)
Knowledge on APBI after OBP is even more limited [94–96]. APBI has proven to be a viable alternative for treating early-stage breast cancer, with results matching those of WBRT [97–99], rendering a revolutionary approach to breast radiation with significant benefits compared with WBRT. Not only does it limit the amount of tissue affected by irradiation and reduce heart, lung, and skin exposure levels, but it also shortens total treatment time from several weeks to 4–5 days. APBI could provide improved outcomes for many patients [96]. Nevertheless, these targeted approaches rely on precise tumour bed delineation, same as WBRT with an additional tumour bed boost. Radiation oncologists usually rely on surgical incisions, postoperative seromas, and clips to determine the tumour bed. Unfortunately, these commonly used methods are not reliable in the setting of OBP techniques such as mammoplasty. Unlike sBCS, in which incisions are sometimes made directly over the lumpectomy cavity, and seromas can form for accurate measurement, tissue flaps from other areas in the breast may be rotated into these cavities, which could displace clinical margins away from their original site. Furthermore, due to a lack of agreement on clip placement practices after BCS, there is a significant variance in the results that further complicate radiation deliniation.
3.3.3. Surgical clips – a flying dutchman for radiation after OBP?
In light of recent developments on breast cancer treatment, a noteworthy Canadian task force has published a set of approaches for pinpointing the tumour bed prior to radiation therapy following oncoplastic procedures [100]. To ensure optimal performance in this area, they recommended that at least four clips be used after OBP; these should be located on the cavity sidewalls (medial, lateral, superior, and inferior) and potentially 1–4 extra clips for posterior margins (which may or may not include the chest wall). Nevertheless, intra- and inter-observer variability can be high when defining a tumoural bed after OBP. A recent study of Aldosary et al. [101] reported compelling findings regarding the accuracy of post-OBP surgical clips as radiographic surrogates for tumoural beds. The volumes, positions, and contours delineated by radiation oncologists indicated significant differences compared with the actual boundaries of the tumour bed. This research revealed that even a large number of surgical clips were insufficient to effectively record the complex, three-dimensional shape changes occurring in breast tissue after oncoplastic surgery.
In light of these data, further investigation is needed to determine the advantages of boost or APBI after OBP, considering the greater radiation exposure and risk of damaging normal breast tissue [100]. WBRT may then be preferable to APBI in complex OBP cases, where accurate targeting is critical for optimal cancer treatment.
Radiotherapy planning after OBP poses particular challenges, and radiation oncologists must consider various factors when treating patients undergoing OBP to ensure optimal treatment outcomes. The modified anatomy may interfere not only with the tumour’s location but also modify what would have been defined as its target area for boost [102,103], making precise volume delineation with accurate dose delivery an arduous task for radiation oncologists, in which marker clips are crucial [103]. To this end, radiation technologists, radiotherapists, and surgeons should strive to develop a shared understanding at their institutions concerning these treatments to better serve those affected by breast cancer. Various solutions, such as using radio-opaque wire markers during surgery, providing comprehensive surgical reports, and educating radiologists more extensively on OBP, can be implemented to optimally identify tumour volumes, enhancing contouring accuracy and improving patient outcomes [100].
3.4. QoL and cosmetic outcomes
Quality care for cancer patients is holistic, addressing the person’s dignity, respect, and needs while maintaining their social, emotional, and intimate well-being. Finding a balance between these factors can be an extensive journey, but providing individuals with opportunities to participate in decisions regarding therapeutic plans and empowering them to regain control of their lives is an essential step in the healing process [104].
Breast cancer treatment often results in several physical effects, which may be temporary or permanent. Chemotherapy side-effects such as hair loss and dental damage tend to dissipate with time, but breast deformity can lead to lasting asymmetry [105] and impact the overall QoL [106]. OBP can be a viable alternative to mastectomy for large and multifocal tumours: although devised to achieve better aesthetic and functional results when compared to sBCS, patients may be subjected to unnecessary overtreatments due to insufficient consideration of their preferences and surgeon’s overuse of these techniques.
between how specialists evaluate cosmetic outcomes and patients opinion [107–110]. In some instances, aesthetic results with sBCS are better rated than those with OBP [111], rendering these, such as reduction mammoplasties for small-sized tumours, as unnecessary and “overtreatment”, without adding value to one’s QoL [24,86,112]. Conversely, some patients aim for “breast symmetry”, which sometimes can only be achieved using oncoplastic techniques.
After assessing patient-reported outcomes and cosmetic evaluations, a meta-analysis was not possible in the latest Cochrane review [83]. In general OBP patients reported comparable or more positive results than sBCS patients – but data is inconclusive due to potential bias in measurement techniques used and with a high heterogeneity amongst the used questionnaires (Breast-Q, EORTC breast questionnaire, Breast Cancer Treatment Outcome Scale, 36-Item Short Form Survey, Rosenberg-EPM Self-Esteem Scale and self-designed unvalidated questionnaires). Additionally, the Cochrane panel assessment suggested an aesthetic benefit from OBP; however, this outcome was hampered by risk bias due to measurement methods used in the analysed studies (some studies used the computer programme BCCT.core, but the majority used an expert panel and self-designed non-validated assessment scales). The iTOP trial [113], a small but prospective study, compared QoL among 205 patients who underwent sBCS, OBP, and mastectomy with immediate reconstruction. At 12 months after the primary surgery, self-esteem was evaluated using a body image scale (BIS) and BREAST-Q questionnaire. The results revealed no statistically significant differences between the studied groups. However, a comparative analysis between the OBP and sBCS groups revealed that the tumours in the former group were larger.
Oncoplastic surgery, requires detailed evaluation with precise and validated standardised tools to provide valuable insight into the effectiveness of OBP, from both clinical perspective and patient-reported experience. Nevertheless, objective measurement of the intricate interplay of psychological and surgical-specific issues affecting the QoL of patients with breast cancer is limited by the lack of validated tools and metrics available for use in clinical practice [114,115]. Thus, to improve outcomes in this area, existing tools should be improved upon to enhance data accuracy and reliability in terms of QoL and aesthetic outcomes [14]. Moreover, the reviewed literature suggests that using author-generated questionnaires, often not validated to measure outcomes, makes it even more difficult to compare and pool data between studies [116]. In addition, there is a pressing need for further studies to gain insight into patient outcomes following immediate or delayed contralateral symmetry surgery. Also, it would be beneficial to gain more knowledge on the rate of revision surgeries necessary due to aesthetic issues caused by OBP [88]. Therefore, it might be wise for breast surgeons to exercise caution when using extensive, complicated OBP approaches that might not significantly improve patients’ chances of survival or QoL [117].
3.4.1. The perfect tool (how cosmesis influences patient psychological outcome)
Aesthetics is continually shaped and reshaped by personal, professional, and social influences. Immanuel Kant argued that beauty lies in the eye of the beholder – judgement being formed through prior experiences rather than an absolute truth or global standard [118]. Aesthetic outcomes in the realm of breast surgery can be a complex balancing act with numerous variables to consider. Breast reconstruction surgery is an emotional and important process for women, impacting their self-image. Nevertheless, patients often have varying preferences when it comes to decision-making, from active involvement in their treatment to passive roles. Regardless of the chosen style, it is beneficial to include education and appearance counselling in the decision-making process to ensure positive emotional and physical outcomes. Appearance counselling promotes realistic expectations regarding what one might look like after breast surgery.
Despite conventional approaches, uncertainty still leads to dissatisfaction with decisions, but utilising modern techniques such as machine learning and artificial intelligence (AI) tools might help overcome decision-related regrets and enhance realistic outcome expectations (see Table 2). AI algorithms can quickly and accurately analyse large datasets, making them valuable in medical decision. In addition, AI can identify patterns within the data that may not be evident to the human eye. This could significantly improve patient care by providing clinicians with more reliable and objective evaluations of OBP outcomes, with detailed information about aesthetic results, body image issues, and other areas of concern after surgery. Although further research is needed to explore the efficacy of these tools in clinical practice, the potential of this technology is undeniable. With continued development and refinement, AI may soon become a valuable tool for improving outcomes evaluation in oncoplastic breast surgery.

3.5. The cost
OBP are more challenging than sBCS, in some cases requiring multiple stage reconstruction or flap-based reconstruction, and can be significantly more expensive. Although improving health is paramount, it is equally important to consider costs when assessing different medical treatments. Doing so helps individuals and society make the most of their resources and limits the economic impact incurred by out-of-pocket expenses when the procedures are not reimbursed or lost wages due to additional work time off during treatments [122,123].
An analysis of the American College of Surgeons National Surgical Quality Improvement Program database has shown that in breast cancer patients with moderate to large-size breasts, using OBP in the BCS context could be a more cost-effective approach compared to mastectomy and implant or free flap reconstruction techniques, making it an attractive prospect for women and systems who wish to reduce healthcare expenses without sacrificing quality care [76,124,125]. A single-centre retrospective cohort study from Europe [126] evaluated the costs of sBCS, OBP, and implant-based (without acellular dermal matrix) immediate-delayed breast reconstruction. It was concluded that OBP were significantly more expensive than sBCS. After analysing 220 patients over a follow-up period of 18 months, the authors reported a mean total cost of approximately 12,000 euros/patient for sBCS and about 14,000 euros/patient for OBP and implant-based reconstructions. However, the complication rates were significantly higher in the implant-based reconstruction group than in the sBCS and OBP groups. No significant cost differences between the OPB and implant-based breast reconstruction groups were found because postoperative radiotherapy influenced the total costs for breast conservation treatments. Adjuvant radiotherapy could make up for over 40% of the expenses incurred by breast-conserving treatment. Furthermore, the research excluded work absences and additional costs; therefore, the cost per patient recorded is likely a conservative amount. Comparative analysis of BCS and mastectomy with implant-based reconstruction revealed that patients who underwent BCS had a noticeably faster return to work and social activities and reduced work downtime [127]. Moving forward, a comparative study between the costs and associated benefits of various OBP procedures could deepen our understanding even further. Even more, to capture the full impact of care, an integrated societal perspective must consider not only direct costs to patients but also indirect financial burden.
In many European countries, reimbursement for breast cancer surgery has not kept up with the evolution of oncoplastic and reconstructive surgery. Some OBP and reconstructive surgeries are not fully reimbursed, discouraging providers from offering OBP and leading to limited options and sometimes inappropriate care. Furthermore, without access to oncoplastic and reconstruction services, patients are often forced towards monodisciplinary private surgical practices that lack accountability and quality assurance. The resulting costs can be both economically and clinically high [128].
It is crucial to consider both financial toxicities and aesthetic/clinical outcomes when deciding between sBCS and OBP procedures for patients with breast cancer. Each approach has its own benefits and drawbacks, which should be considered to ensure quality care. While oncoplastic surgery can provide a better aesthetic outcome, it may not be appropriate for those unable to cover the higher costs associated with this type of procedure. Further research is necessary to determine the cost-effectiveness of these procedures and to provide guidance for patients in decision making on their best possible care.
4. SOMETIMES IS BETTER TO JUST MAKE IT SIMPLE. A PROPOSED ALGORITHM TO CHOOSE BETWEEN sBCS AND OBP
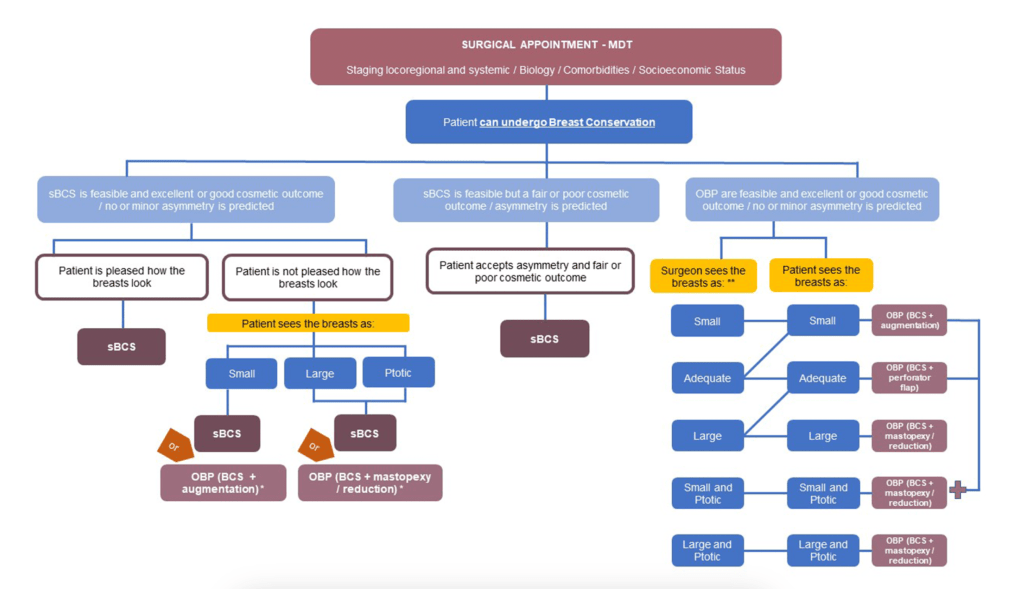
Within the patient journey, most early breast cancer patients will face the surgeon and discuss a surgery-related proposal. This is a stressful moment due to cancer-related fear, the intervention itself, and all uncertainties that can arise after discussing the pros and cons of choosing one option over other. If some cases are easy to decide, many are not; it is the surgeon’s duty to guide the patient through a proper shared decision-making process (see Fig. 1).
5. CONCLUSION
Oncoplastic surgery developed as a new approach to breast cancer, allowing surgeons to offer optimal patient care, and seems established as the preferred surgical treatment for breast cancer. Evidence suggests that OBP and sBCS are similar in terms of LR, DFS, and OS. The need for re-excision may be lower for OBP, but might result in more postoperative complications and possibly more mastectomies. Though evidence is limited in quality, patients and doctors appear to have higher satisfaction levels with OBP aesthetic when compared to sBCS. However, we must keep in mind that OBP can lead to overtreatment of patients who would otherwise been satisfactorily managed by sBCS.
With ongoing medical and scientific advancements, we can now offer better breast cancer treatments targeting specific molecular characteristics to de-escalate systemic therapies. To ensure best possible outcomes, surgical approaches should also be tailored to each individual, to neoadjuvant systemic therapy-induced tumour response allowing in many cases to downstage complex surgical procedures deemed unnecessary or overly cosmetic invasive. Although the “Holy Grail” of surgical treatment de-escalation, embodied by the total omission of invasive procedures (129, 130), seems feasible and reachable, it is still far from real, and surgery remains a key part of breast cancer treatment. De-escalated surgical techniques could provide better aesthetic outcomes with less postoperative morbidity and reduce the need for symmetrisation. In conclusion, we must ensure that OBP is cautiously used until adequate data (clinical and financial), dedicated training and standardised tools to predict aesthetic outcome are available.
FUNDING
Eduard-Alexandru Bonci’s work has been supported by a UICC Technical Fellowship [grant number UICC-TF/2022-1429].
ETHICAL APPROVAL
Not required.
DECLARATION OF COMPETING INTEREST
All authors have no conflict of interest disclosures to report.
ACKNOWLEDGMENTS
The preparation of this study was funded in part by a UICC Technical Fellowship [grant number UICC-TF/2022-1429].
REFERENCES
[1] Breast cancer: the international agency for research on cancer (IARC) [cited 2022 December 26th]. Available from: https://www.iarc.who.int/cancer-type/breast-cancer/.
[2] World Health O. WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva: World Health Organization; 2020. 2020.
[3] Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015;373(4):317–27.
[4] Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378(9804):1707–16.
[5] Jonczyk MM, Jean J, Graham R, Chatterjee A. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat 2019;173(2):267–74.
[6] Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347(16):1227–32.
[7] Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347(16):1233–41.
[8] Blichert-Toft M, Nielsen M, Düring M, Møller S, Rank F, Overgaard M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 2008;47(4):672–81.
[9] Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149(3):267–74.
[10] van Hezewijk M, Bastiaannet E, Putter H, Scholten AN, Liefers GJ, Rea D, et al. Effect of local therapy on locoregional recurrence in postmenopausal women with breast cancer in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. Radiother Oncol 2013;108(2):190–6.
[11] de Boniface J, Szulkin R, Johansson ALV. Survival after breast conservation vs mastectomy adjusted for comorbidity and socioeconomic status: a Swedish national 6-year follow-up of 48 986 women. JAMA Surg 2021;156(7):628–37.
[12] van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. Jnci-J National Cancer Inst 2000;92(14):1143–50.
[13] van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in The Netherlands: a population-based study. Lancet Oncol 2016;17(8):1158–70.
[14] Rocco N, Catanuto G, Cinquini M, Audretsch W, Benson J, Criscitiello C, et al. Should oncoplastic breast conserving surgery be used for the treatment of early stage breast cancer? Using the GRADE approach for development of clinical recommendations. Breast 2021;57:25–35.
[15] Kenny P, King MT, Shiell A, Seymour J, Hall J, Langlands A, et al. Early stage breast cancer: costs and quality of life one year after treatment by mastectomy or conservative surgery and radiation therapy. Breast 2000;9(1):37–44.
[16] Fung KW, Lau Y, Fielding R, Or A, Yip AW. The impact of mastectomy, breast-conserving treatment and immediate breast reconstruction on the quality of life of Chinese women. ANZ J Surg 2001;71(4):202–6.
[17] Ohsumi S, Shimozuma K, Morita S, Hara F, Takabatake D, Takashima S, et al. Factors associated with health-related quality-of-life in breast cancer survivors: influence of the type of surgery. Jpn J Clin Oncol 2009;39(8):491–6.
[18] Kühn T. Determinant of long-term quality of life: surgery in breast cancer treatment. Breast Care 2012;7(5):361–2.
[19] Sun Y, Kim SW, Heo CY, Kim D, Hwang Y, Yom CK, et al. Comparison of quality of life based on surgical technique in patients with breast cancer. Jpn J Clin Oncol 2014;44(1):22–7.
[20] Nano MT, Gill PG, Kollias J, Bochner MA, Malycha P, Winefield HR. Psychological impact and cosmetic outcome of surgical breast cancer strategies. ANZ J Surg 2005;75(11):940–7.
[21] Yurek D, Farrar W, Andersen BL. Breast cancer surgery: comparing surgical groups and determining individual differences in postoperative sexuality and body change stress. J Consult Clin Psychol 2000;68(4):697–709.
[22] Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol 2008;26(20):3331–7.
[23] Clough KB, Ihrai T, Oden S, Kaufman G, Massey E, Nos C. Oncoplastic surgery for breast cancer based on tumour location and a quadrant-per-quadrant atlas. Br J Surg 2012;99(10):1389–95.
[24] Silverstein MJ, Mai T, Savalia N, Vaince F, Guerra L. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol 2014;110(1):82–9.
[25] Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72 (2):145–9.
[26] De La Cruz L, Blankenship SA, Chatterjee A, Geha R, Nocera N, Czerniecki BJ, et al. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann Surg Oncol 2016;23(10):3247–58.
[27] Clough KB, Cuminet J, Fitoussi A, Nos C, Mosseri V. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg 1998;41(5):471–81.
[28] D’Aniello C, Grimaldi L, Barbato A, Bosi B, Carli A. Cosmetic results in 242 patients treated by conservative surgery for breast cancer. Scand J Plast ReConstr Surg Hand Surg 1999;33(4):419–22.
[29] Matory WE, Wertheimer M, Fitzgerald TJ, Walton RL, Love S. Aesthetic results following partial mastectomy and radiation therapy. Plast Reconstr Surg 1990;85 (5):739–46.
[30] Chatterjee A, Gass J, Patel K, Holmes D, Kopkash K, Peiris L, et al. A consensus definition and classification system of oncoplastic surgery developed by the American society of breast surgeons. Ann Surg Oncol 2019;26(11):3436–44.
[31] Gilmour A, Cutress R, Gandhi A, Harcourt D, Little K, Mansell J, et al. Oncoplastic breast surgery: a guide to good practice. Eur J Surg Oncol 2021;47(9):2272–85.
[32] Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17(5):1375–91.
[33] Carter SA, Lyons GR, Kuerer HM, Bassett RL, Oates S, Thompson A, et al. Operative and oncologic outcomes in 9861 patients with operable breast cancer: single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol 2016;23(10):3190–8.
[34] Niinikoski L, Leidenius MHK, Vaara P, Voynov A, Heikkil¨a P, Mattson J, et al. Resection margins and local recurrences in breast cancer: comparison between conventional and oncoplastic breast conserving surgery. Eur J Surg Oncol 2019; 45(6):976–82.
[35] Weber WP, Soysal SD, El-Tamer M, Sacchini V, Knauer M, Tausch C, et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res Treat 2017;165(1):139–49.
[36] Clough KB, van la Parra RFD, Thygesen HH, Levy E, Russ E, Halabi NM, et al. Long-term results after oncoplastic surgery for breast cancer: a 10-year follow-up. Ann Surg 2018;268(1):165–71.
[37] Franceschini G, Terribile D, Magno S, Fabbri C, Accetta C, Di Leone A, et al. Update on oncoplastic breast surgery. Eur Rev Med Pharmacol Sci 2012;16(11): 1530–40.
[38] Franceschini G, Magno S, Fabbri C, Chiesa F, Di Leone A, Moschella F, et al. Conservative and radical oncoplastic approches in the surgical treatment of breast cancer. Eur Rev Med Pharmacol Sci 2008;12(6):387–96.
[39] Masetti R, Di Leone A, Franceschini G, Magno S, Terribile D, Fabbri MC, et al. Oncoplastic techniques in the conservative surgical treatment of breast cancer: an overview. Breast J 2006;12(5 Suppl 2):S174–80.
[40] Liang Y, Muse-Fisher C, Rambukwella M, Naber SP, Chatterjee A. Malignant and high-risk lesions in the contralateral breast symmetry mastopexy and reduction specimens when performing large-volume displacement oncoplastic surgery. Ann Plast Surg 2019;82(4S Suppl 3):S185–91.
[41] Petit JY, Rietjens M, Contesso G, Bertin F, Gilles R. Contralateral mastoplasty for breast reconstruction: a good opportunity for glandular exploration and occult carcinomas diagnosis. Ann Surg Oncol 1997;4(6):511–5.
[42] Mohamedahmed AYY, Zaman S, Zafar S, Laroiya I, Iqbal J, Tan MLH, et al. Comparison of surgical and oncological outcomes between oncoplastic breast-conserving surgery versus conventional breast-conserving surgery for treatment of breast cancer: a systematic review and meta-analysis of 31 studies. Surg Oncol 2022;42:101779.
[43] Knowles S, Maxwell J, Lumsden A, Pearson L, Pulhin J, McLean J, et al. An alternative to standard lumpectomy: a 5-year case series review of oncoplastic breast surgery outcomes in a Canadian setting. Can J Surg 2020;63(1):E46–51.
[44] Losken A, Hart AM, Broecker JS, Styblo TM, Carlson GW. Oncoplastic breast reduction technique and outcomes: an evolution over 20 years. Plast Reconstr Surg 2017;139(4). 824e-33e.
[45] de Boniface J, Szulkin R, Johansson ALV. Major surgical postoperative complications and survival in breast cancer: Swedish population-based register study in 57 152 women. Br J Surg 2022;109(10):977–83.
[46] Rose M, Manjer J, Ringberg A, Svensson H. Surgical strategy, methods of reconstruction, surgical margins and postoperative complications in oncoplastic breast surgery. Eur J Plast Surg 2014;37(4):205–14.
[47] McCulley SJ, Macmillan RD. Therapeutic mammaplasty–analysis of 50 consecutive cases. Br J Plast Surg 2005;58(7):902–7.
[48] Carstensen L, Bigaard J. Management of central breast tumours with immediate reconstruction of the nipple-areola complex; a suggested guide. Breast 2015;24 (1):38–45.
[49] Aristokleous I, Saddiq M. Quality of life after oncoplastic breast-conserving surgery: a systematic review. ANZ J Surg 2019;89(6):639–46.
[50] Hart AM, Pinell-White X, Egro FM, Losken A. The psychosexual impact of partial and total breast reconstruction: a prospective one-year longitudinal study. Ann Plast Surg 2015;75(3):281–6.
[51] Kabir SA, Stallard S, Weiler-Mithoff E, Mansell J, Mallon E, Doughty JC, et al. Six-year follow-up of patients treated with oncoplastic reduction mammoplasty: a cohort study. Int J Surg 2016;26:38–42.
[52] Resende Paulinelli R, de Oliveira VM, Bagnoli F, Letzkus Berríos J, C´ezar Chade M, Bragatto Picoli L, et al. Oncoplastic mammaplasty with geometric compensation: evolution of the technique, outcomes and follow-up in a multicentre retrospective cohort. J Surg Oncol 2020;121(6):967–74.
[53] Hourston G, Joglekar S, Down S, Downey S, Pereira J. Has the time come for de-escalation in oncoplastic breast conserving surgery? Eur J Surg Oncol 2022;48(2): 309–11.
[54] Wijgman DJ, Ten Wolde B, van Groesen NR, Keemers-Gels ME, van den Wildenberg FJ, Strobbe LJ. Short term safety of oncoplastic breast conserving surgery for larger tumors. Eur J Surg Oncol 2017;43(4):665–71.
[55] Crown A, Wechter DG, Grumley JW. Oncoplastic breast-conserving surgery reduces mastectomy and postoperative Re-excision rates. Ann Surg Oncol 2015; 22(10):3363–8.
[56] Clough KB, Acosta-Marín V, Nos C, Alran S, Rouanet P, Garbay JR, et al. Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer surgery: a French national survey. Ann Surg Oncol 2015;22(11):3504–11.
[57] Kaufman CS. Increasing role of oncoplastic surgery for breast cancer. Curr Oncol Rep 2019;21(12):111. [58] Suzanne Klimberg V. Oncoplastic breast surgery: look good feel better. Eur J Surg Oncol 2021;47(9):2211.
[59] Silverstein MJ. Oncoplastic breast surgery: from oblivion to mainstream. Ann Surg Oncol 2019;26(11):3409–12.
[60] Wyld L, Rubio IT, Kovacs T. Education and training in breast cancer surgery in Europe. Breast Care 2019;14(6):366–72.
[61] Simpson JS, Scheer AS. A review of the effectiveness of breast surgical oncology fellowship programs utilizing kirkpatrick’s evaluation model. J Cancer Educ 2016;31(3):466–71.
[62] Kovacs T, Rubio IT, Markopoulos C, Audisio RA, Knox S, Kühn T, et al. Theoretical and practical knowledge curriculum for European Breast Surgeons. Eur J Surg Oncol 2020;46(4 Pt B):717–36.
[63] Montagna G, Morgan J, Wandschneider W, Vinci A, Esgueva A, Corso G, et al. Implementation of the BRESO Theoretical and practical knowledge curriculum for European Breast Surgeons: the time has come. Eur J Surg Oncol 2020;46(4 Pt B):715–6.
[64] Sanchez AM, Franceschini G, D’Archi S, De Lauretis F, Scardina L, Di Giorgio D, et al. Results obtained with level II oncoplastic surgery spanning 20 years of breast cancer treatment: do we really need further demonstration of reliability? Breast J 2020;26(2):125–32.
[65] Maxwell J, Covelli AM, Scheer A, Roberts A, Osman F, Escallon J, et al. Challenges in utilizing oncoplastic techniques in breast conserving surgery. Breast J 2019;25(3):555–6.
[66] Adamson K, Chavez-MacGregor M, Caudle A, Smith B, Baumann D, Liu J, et al. Neoadjuvant chemotherapy does not increase complications in oncoplastic breast-conserving surgery. Ann Surg Oncol 2019;26(9):2730–7.
[67] Chauhan A, Sharma MM, Kumar K. Evaluation of surgical outcomes of oncoplasty breast surgery in locally advanced breast cancer and comparison with conventional breast conservation surgery. Indian J Surg Oncol 2016;7(4):413–9.
[68] Tang W, Liu J, Yang H, Jiang Y, Wei W, editors. Clinical comparative study of oncoplastic and standard breast-conserving surgery in the treatment of early breast cancer; 2016.
[69] Mustonen P, Lepist¨o J, Papp A, Berg M, Pietil¨ainen T, Kataja V, et al. The surgical and oncological safety of immediate breast reconstruction. Eur J Surg Oncol 2004;30(8):817–23.
[70] Acea-Nebril B. Conservative oncoplastic surgery in breast cancer. Indications and limitations to its application. Cir Esp 2005;78(1):12–8.
[71] Giacalone PL, Roger P, Dubon O, El Gareh N, Rihaoui S, Taourel P, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 2007;14(2):605–14.
[72] Palsdottir EP, Lund SHL, Asgeirsson KSA. Oncoplastic breast-conserving surgery in Iceland: a population-based study. Scand J Surg 2018;107(3):224–9.
[73] Tenofsky PL, Dowell P, Topalovski T, Helmer SD. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Am J Surg 2014;207(3):398–402 [discussion].
[74] Acosta-Marin V, Acosta-Freites V, Contreras A, Ravelo R, Fuenmayor G, Marin C, et al. Oncoplastic breast surgery: initial experience at the Centro Clinico de Estereotaxia-CECLINES, Caracas, Venezuela. Ecancermedicalscience 2014;8:470.
[75] Peled AW, Sbitany H, Foster RD, Esserman LJ. Oncoplastic mammoplasty as a strategy for reducing reconstructive complications associated with postmastectomy radiation therapy. Breast J 2014;20(3):302–7.
[76] Angarita FA, Acuna SA, Cordeiro E, McCready DR, Cil TD. Does oncoplastic surgery increase immediate (30-day) postoperative complications? An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Breast Cancer Res Treat 2020;182(2):429–38.
[77] Shechter S, Friedman O, Inbal A, Arad E, Menes T, Barsuk D, et al. Oncoplastic partial breast reconstruction improves patient satisfaction and aesthetic outcome for central breast tumours. ANZ J Surg 2019;89(5):536–40.
[78] Tong WMY, Baumann DP, Villa MT, Mittendorf EA, Liu J, Robb GL, et al. Obese women experience fewer complications after oncoplastic breast repair following partial mastectomy than after immediate total breast reconstruction. Plast Reconstr Surg 2016;137(3):777–91.
[79] Giacalone PL, Dubon O, Roger P, El Gareh N, Rihaoui S, Daur´es JP. Doughnut mastopexy lumpectomy versus standard lumpectomy in breast cancer surgery: a prospective study. Eur J Surg Oncol 2007;33(3):301–6.
[80] De Lorenzi F, Hubner G, Rotmensz N, Bagnardi V, Loschi P, Maisonneuve P, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol 2016;42(1):71–7.
[81] Zhou L, Wang Y, Cai R, Huang J, Li X, Xie Z, et al. Pedicled descending branch latissimus dorsi mini-flap in repairing partial mastectomy defect: shoulder functional and esthetic outcomes. J Surg Oncol 2019;120(3):518–26.
[82] Ozmen V, Ilgun S, Celet Ozden B, Ozturk A, Aktepe F, Agacayak F, et al. Comparison of breast cancer patients who underwent partial mastectomy (PM) with mini latissimus dorsi flap (MLDF) and subcutaneous mastectomy with implant (M + I) regarding quality of life (QOL), cosmetic outcome and survival rates. World J Surg Oncol 2020;18(1):87.
[83] Nanda A, Hu J, Hodgkinson S, Ali S, Rainsbury R, Roy PG. Oncoplastic breast-conserving surgery for women with primary breast cancer. Cochrane Database Syst Rev 2021;10(10):CD013658.
[84] Gupta S, King WD, Korzeniowski M, Wallace DL, Mackillop WJ. The effect of waiting times for postoperative radiotherapy on outcomes for women receiving partial mastectomy for breast cancer: a systematic review and meta-analysis. Clin Oncol 2016;28(12):739–49.
[85] Borm KJ, Sch¨onknecht C, Nestler A, Oechsner M, Waschulzik B, Combs SE, et al. Outcomes of immediate oncoplastic surgery and adjuvant radiotherapy in breast cancer patients. BMC Cancer 2019;19(1):907.
[86] Clough KB, Gouveia PF, Benyahi D, Massey EJ, Russ E, Sarfati I, et al. Positive margins after oncoplastic surgery for breast cancer. Ann Surg Oncol 2015;22(13): 4247–53.
[87] Heeg E, Jensen MB, H¨olmich LR, Bodilsen A, Tollenaar RAEM, Laenkholm AV, et al. Rates of re-excision and conversion to mastectomy after breast-conserving surgery with or without oncoplastic surgery: a nationwide population-based study. Br J Surg 2020;107(13):1762–72.
[88] Losken A, Chatterjee A. Improving results in oncoplastic surgery. Plast Reconstr Surg 2021;147(1). 123e-34e.
[89] Amabile MI, Mazouni C, Guimond C, Sarfati B, Leymarie N, Cloutier AS, et al. Factors predictive of Re-excision after oncoplastic breast-conserving surgery. Anticancer Res 2015;35(7):4229–34.
[90] Herskind C, Ma L, Liu Q, Zhang B, Schneider F, Veldwijk MR, et al. Biology of high single doses of IORT: RBE, 5 R’s, and other biological aspects. Radiat Oncol 2017;12(1):24.
[91] Faverly DR, Hendriks JH, Holland R. Breast carcinomas of limited extent: frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer 2001;91(4):647–59.
[92] van Limbergen E, van den Bogaert W, van der Schueren E, Rijnders A. Tumor excision and radiotherapy as primary treatment of breast cancer. Analysis of patient and treatment parameters and local control. Radiother Oncol 1987;8(1): 1–9.
[93] van Mourik AM, Elkhuizen PH, Minkema D, Duppen JC, van Vliet- Vroegindeweij C, Group DYBS. Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines. Radiother Oncol 2010;94(3):286–91.
[94] Roth AM, Kauer-Dorner D, Resch A, Schmid A, Thill M, Niehoff P, et al. Is oncoplastic surgery a contraindication for accelerated partial breast radiation using the interstitial multicatheter brachytherapy method? Brachytherapy 2014; 13(4):394–9.
[95] Major T, Guti´errez C, Guix B, van Limbergen E, Strnad V, Polg´ar C, et al. Recommendations from GEC ESTRO Breast Cancer Working Group (II): target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving open cavity surgery. Radiother Oncol 2016;118(1):199–204.
[96] Strnad V, Hannoun-Levi JM, Guinot JL, L¨ossl K, Kauer-Dorner D, Resch A, et al. Recommendations from GEC ESTRO Breast Cancer Working Group (I): target definition and target delineation for accelerated or boost Partial Breast Irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother Oncol 2015;115(3):342–8.
[97] Vicini F, Shah C, Arthur D, Khan A, Wazer D, Keisch M. Partial breast irradiation and the GEC-ESTRO trial. Lancet 2016;387(10029):1717–8.
[98] Vicini FA, Cecchini RS, White JR, Arthur DW, Julian TB, Rabinovitch RA, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019;394(10215):2155–64.
[99] Livi L, Meattini I, Marrazzo L, Simontacchi G, Pallotta S, Saieva C, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51(4):451–63.
[100] Tse T, Knowles S, B´elec J, Caudrelier JM, Lock M, Brackstone M, et al. Consensus statement on tumour bed localization for radiation after oncoplastic breast surgery. Curr Oncol 2020;27(3):e326–31.
[101] Aldosary G, Caudrelier JM, Arnaout A, Chang L, Tse T, Foottit C, et al. Can we rely on surgical clips placed during oncoplastic breast surgery to accurately delineate the tumor bed for targeted breast radiotherapy? Breast Cancer Res Treat 2021; 186(2):343–52.
[102] Eaton BR, Losken A, Okwan-Duodu D, Schuster DM, Switchenko JM, Mister D, et al. Local recurrence patterns in breast cancer patients treated with oncoplastic reduction mammaplasty and radiotherapy. Ann Surg Oncol 2014;21(1):93–9.
[103] Pezner RD. The oncoplastic breast surgery challenge to the local radiation boost. Int J Radiat Oncol Biol Phys 2011;79(4):963–4.
[104] Organization WH. Strengthening of palliative care as a component of integrated treatment throughout the life course. J Pain Palliat Care Pharmacother 2014;28 (2):130–4.
[105] Grujic D, Giurgi-Oncu C, Oprean C, Cr˘ainiceanu Z, Secoșan I, Riviș I, et al. Well-being, depression, and anxiety following oncoplastic breast conserving surgery versus modified radical mastectomy followed by late breast reconstruction. Int J Environ Res Publ Health 2021;18(17).
[106] Rose M, Svensson H, Handler J, Hoyer U, Ringberg A, Manjer J. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res Treat 2020;180(1): 247–56.
[107] Eichler C, Kolsch M, Sauerwald A, Bach A, Gluz O, Warm M. Lumpectomy versus mastopexy–a post-surgery patient survey. Anticancer Res 2013;33(2):731–6.
[108] Santos G, Urban C, Edelweiss MI, Zucca-Matthes G, de Oliveira VM, Arana GH, et al. Long-term comparison of aesthetical outcomes after oncoplastic surgery and lumpectomy in breast cancer patients. Ann Surg Oncol 2015;22(8):2500–8.
[109] Kim MK, Kim T, Moon HG, Jin US, Kim K, Kim J, et al. Effect of cosmetic outcome on quality of life after breast cancer surgery. Eur J Surg Oncol 2015;41(3): 426–32.
[110] Bertozzi N, Pesce M, Santi PL, Raposio E. Oncoplastic breast surgery: comprehensive review. Eur Rev Med Pharmacol Sci 2017;21(11):2572–85.
[111] Acea-Nebril B, García-Novoa A, Cereijo-Garea C. Cosmetic sequelae after oncoplastic breast surgery: long-term results of a prospective study. Breast J 2021; 27(1):35–43.
[112] Tan MP. Minimalist breast conserving surgical approaches for inferiorly sited cancers. Gland Surg 2017;6(4):399–409.
[113] Bolliger M, Lanmüller P, Schuetz M, Heilig B, Windischbauer A, Jakesz R, et al. The iTOP trial: comparing immediate techniques of oncoplastic surgery with conventional breast surgery in women with breast cancer – a prospective, controlled, single-center study. Int J Surg 2022;104:106694.
[114] Rutherford CL, Barker S, Romics L. A systematic review of oncoplastic volume replacement breast surgery: oncological safety and cosmetic outcome. Ann R Coll Surg Engl 2022;104(1):5–17.
[115] Scomacao I, AlHilli Z, Schwarz G. The role of oncoplastic surgery for breast cancer. Curr Treat Options Oncol 2020;21(12):94.
[116] Raufdeen F, Murphy J, Ahluwalia M, Coroneos CJ, Thoma A. Outcomes in volume replacement and volume displacement techniques in oncoplastic breast conserving surgery: a systematic review. J Plast Reconstr Aesthetic Surg 2021;74 (11):2846–55.
[117] Catanuto G, Khan A, Ursino V, Pietraforte E, Scandurra G, Ravalli C, et al. De-escalation of complexity in oncoplastic breast surgery: case series from a specialized breast center. Breast 2019;46:12–8.
[118] Kant I, Guyer P. Critique of the power of judgment. Cambridge: Cambridge University Press; 2000. [119] Kydd LA, Reid SA, Adams J. The Breast Surgery Gallery: an educational and counseling tool for people with breast cancer or having prophylactic breast surgery. Clin J Oncol Nurs 2010;14(5):643–8.
[120] Nicklaus KM, Cheong A, Sampathkumar U, Liu J, Chopra D, Hoffman A, et al. Breast decisions: recommender system for appearance counseling about breast reconstruction. Plast Reconstr Surg Glob Open 2022;10(11):e4615.
[121] Champalimaud F. Comparing decision on aesthetics after breast cancer locoregional treatment. 2022. https://ClinicalTrials.gov/show/NCT05196269.
[122] Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 2011;103(2): 117–28.
[123] Sheckter CC, Matros E, Lee GK, Selber JC, Offodile AC. Applying a value-based care framework to post-mastectomy reconstruction. Breast Cancer Res Treat 2019;175(3):547–51.
[124] Asban A, Homsy C, Chen L, Fisher C, Losken A, Chatterjee A. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with single stage implant reconstruction in the treatment of breast cancer. Breast 2018;41:159–64.
[125] Chatterjee A, Asban A, Jonczyk M, Chen L, Czerniecki B, Fisher CS. A cost-utility analysis comparing large volume displacement oncoplastic surgery to mastectomy with free flap reconstruction in the treatment of breast cancer. Am J Surg 2019; 218(3):597–604.
[126] Witmer TJK, Kouwenberg CAE, Bargon CA, de Leeuw DM, Koiter E, Siemerink EJM, et al. Comparing costs of standard breast-conserving surgery to oncoplastic breast-conserving surgery and mastectomy with immediate two-stage implant-based breast reconstruction. J Plast Reconstr Aesthetic Surg 2022;75(8): 2569–76.
[127] Kelsall JE, McCulley SJ, Brock L, Akerlund MTE, Macmillan RD. Comparing oncoplastic breast conserving surgery with mastectomy and immediate breast reconstruction: case-matched patient reported outcomes. J Plast Reconstr Aesthetic Surg 2017;70(10):1377–85.
[128] Cardoso F, MacNeill F, Penault-Llorca F, Eniu A, Sardanelli F, Nordstr¨om EB, et al. Why is appropriate healthcare inaccessible for many European breast cancer patients? – the EBCC 12 manifesto. Breast 2021;55:128–35.
